Digital treatment support for all oncological indications
Mika is a digital and cross-indication assistant to support people receiving treatment for cancer.
Several clinical studies have already shown that using Mika has a positive effect on the course of treatment.
Contact us
Our partners
The Mika app was developed in collaboration with leading research institutions.
All content undergoes rigorous expert review before publication.
All content undergoes rigorous expert review before publication.



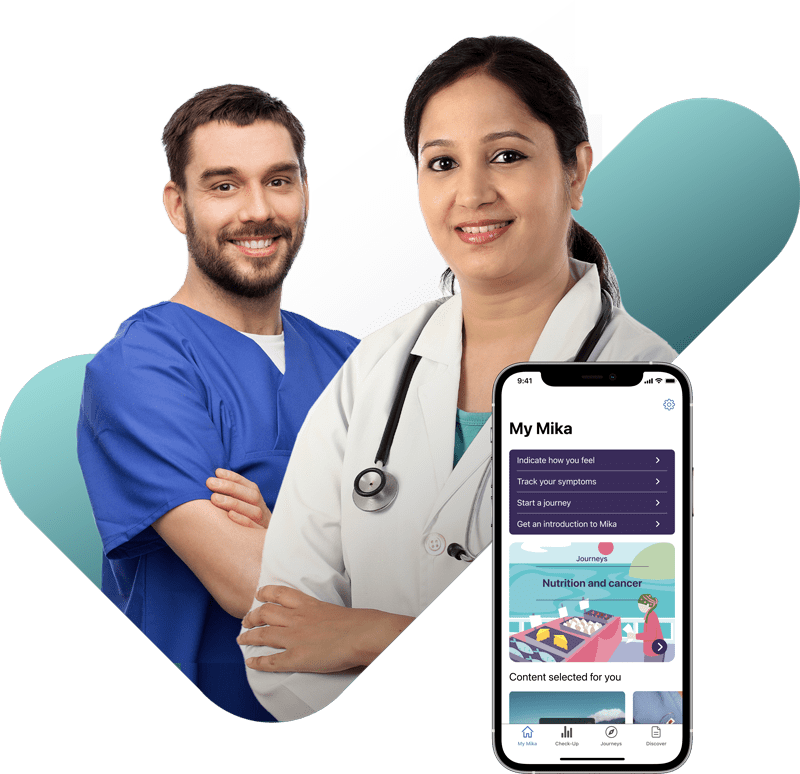
How Mika can support your patients and your practice
Mika during consultations
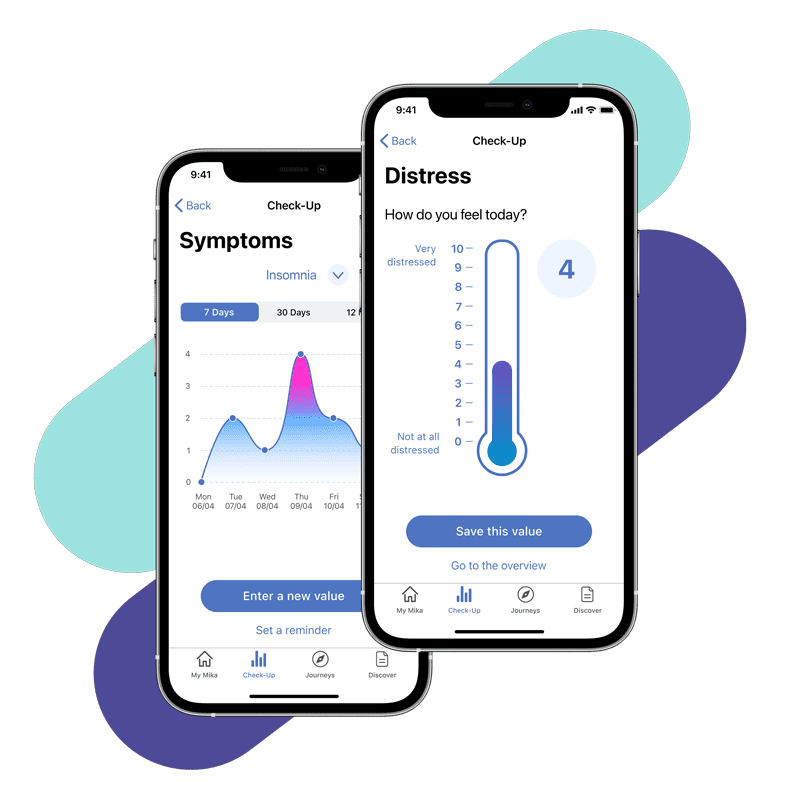
Mika makes it quick and easy for patients to monitor their symptoms and any side effects. This ensures patients are better prepared for their consultation and can bring a precise record of symptoms with them.
- Gain an overview faster
- Make more informed decisions thanks to accurate data
- Win time to better tailor treatment to your patients’ needs
Mika in everyday use by patients
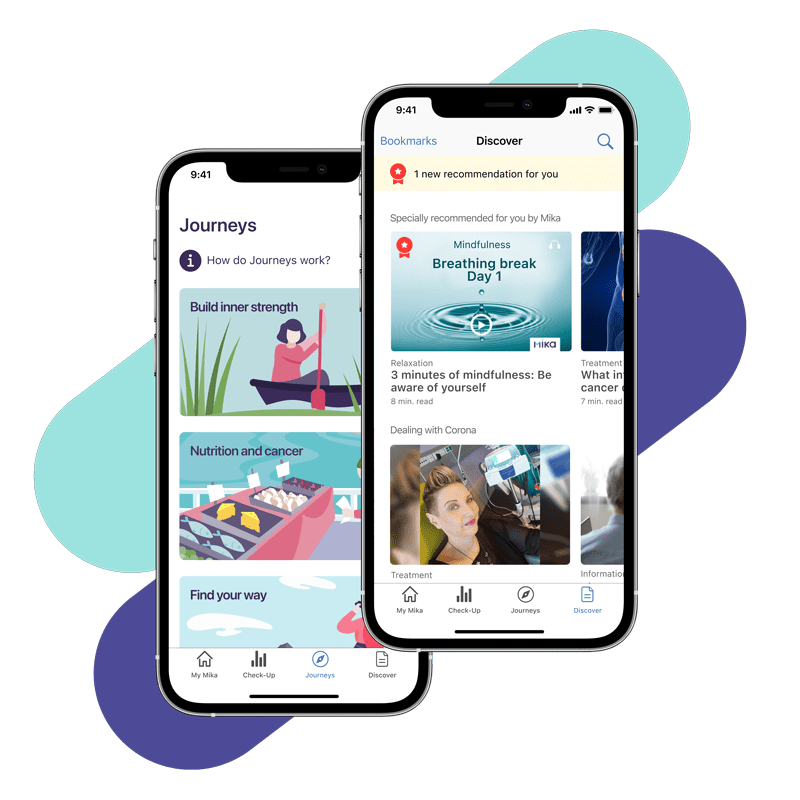
Mika offers evidence-based, guideline-compliant and validated content designed to enhance quality of life. The app contains tips and information on nutrition, exercise and relaxation, but also on socio-legal and oncological matters. All content is presented in an easy-to-understand way and is fully accessible.
- Provides only correct information
- Low-threshold educational content promotes use
- Practical tips make everyday life easier
Effectiveness and research
Pilot study conducted at the Charité - Positive effects of supporting treatment with Mika
-0%
Fatigue1
-0%
Psychological distress1
1 Prospective Randomized Controlled Pilot Study of the Clinical Efficacy of the Fosanis Mika App 2020
A 12-week prospective, randomised and controlled pilot study conducted at the Charité Berlin sought to establish the effects of supporting cancer treatment with Mika.
The probands of the pilot study were patients with gynecological tumours.
Patients who used Mika reported a 23% reduction of fatigue and a 42% reduction of psychological distress after a 12-week period of app-based support in comparison to before they used the app.
The clinical validity of the observed effects was found to be sufficient (Cohen's d).
OnkoDigiTrial II
Comprehensive intervention effects on distress-associated parameters
Partner
University Hospital Leipzig, Germany
Principal Investigator
Prof. Dr. Anja Mehnert-Theuerkauf
Study design
Randomised controlled trial:
12 weeks Mika app; waiting list control group
12 weeks Mika app; waiting list control group
Sample size
218 Patients with a variety of tumours (ICD-10: C00-C97)
Clinical endpoints
Primary: change in psychological distress (DT).
Secondary/tertiary: changes in fatigue (FACIT-F), depression and anxiety (HADS-D), quality of life (CGI-I, SF-8), adherence, patient sovereignty, health literacy
Secondary/tertiary: changes in fatigue (FACIT-F), depression and anxiety (HADS-D), quality of life (CGI-I, SF-8), adherence, patient sovereignty, health literacy
The randomised controlled trial (RCT) showed comprehensive intervention effects on a number of distress-associated parameters after 12 weeks of using the Mika app - compared to the control group with standard care:
- Reduction of psychological distress
- Reduction of depressive symptoms
- Reduction of anxiety symptoms
- Reduction of fatigue
The digital Mika offers clinically effective psycho-oncological treatment support that can help to improve the mental health and wellbeing of cancer patients.
Information for you and your staff
Would you like to know more about Mika? Just fill out the form below and we'll take care of it!
We are here to help!
Are you a doctor, psychotherapist or oncology specialist and have questions about the Mika app?
Simply contact us directly via: hcp@mika.health
CLINICAL RESEARCH
Mika has been proven to be clinically effective.Medical device
Mika is a Medical Device in accordance with Medical Device Regulation (EU) 2017/745.SECURITY
We comply with rigorous data protection guidelines.







